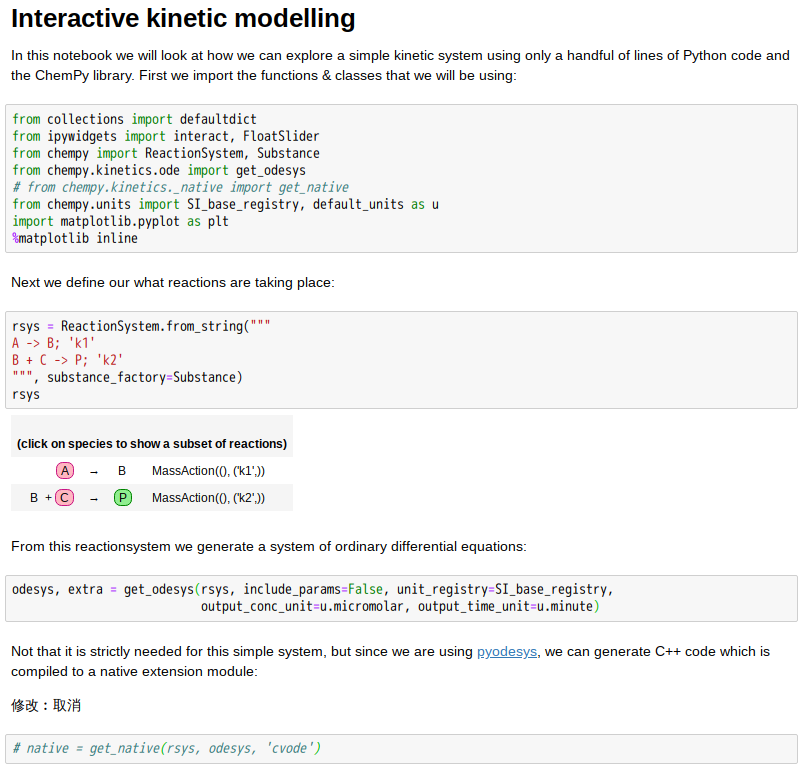
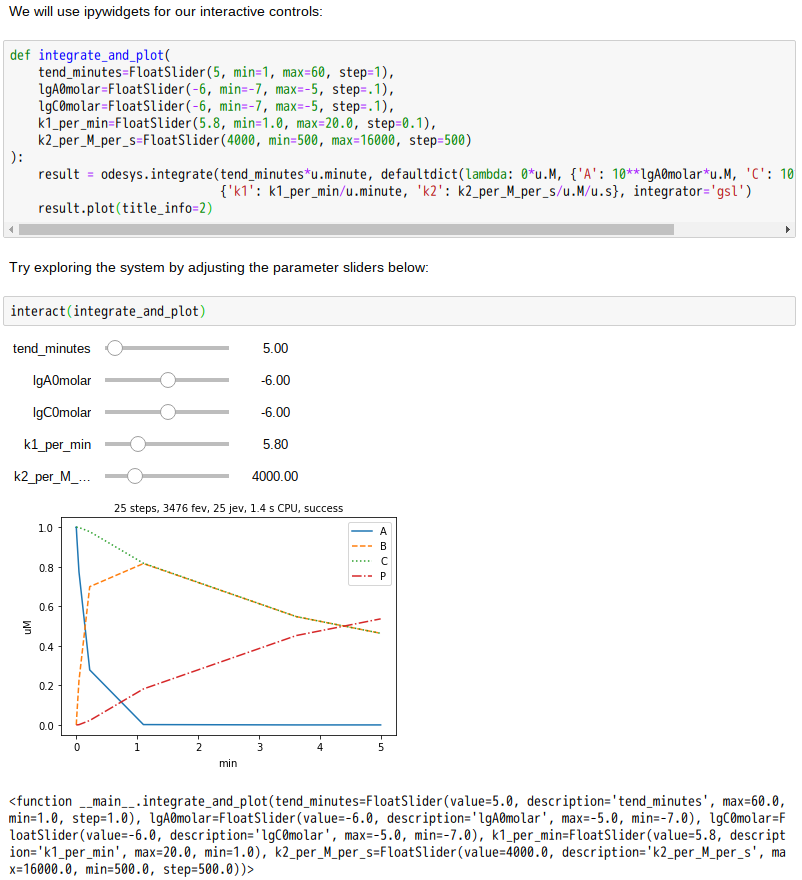
先秦天子的冕服有許多象徵意義,上衣表天、象以日夜,故色為之青黑,下裳示地,徵之富饒,用黃中透紅之彩。十二章紋述說一年,上下均分說著夏冬,其數各六實乃是周易的乾坤。古代上衣下裳的形制,因能方便耕作勞動,也就變成大眾的傳統服飾了。
古之讀書人常有配戴『玉珮』的習慣,這是因為──
《禮記‧聘義》
子貢問於孔子曰︰敢問君子貴玉而賤媒者,何也?為玉之寡而媒之多與?孔子曰︰非為媒之多,故賤之也;玉之寡,故貴之也。夫昔者,君子比德於玉焉,溫潤而澤、仁也,縝密以栗、知也,廉而不劌、義也,垂之如隊、禮也,叩之其聲清越以長其終詘然 、樂也,瑕不掩瑜、瑜不掩瑕、忠也,孚尹旁達、信也,氣如白虹、天也,精神見於山川、地也,圭璋特達、德也。天下莫不貴者、道也。詩云︰言念君子.溫其如玉,故君子貴之也。──。
……
周易經傳在數千年的歷史中,不知被『結構』和『解構』過多少次 ,這是為什麼呢?或許是川流不息的生命之河,自有它亙古的奧秘,也是人類永恆的追求!!誰又能知有沒有『因明』的一天呢? ?如果『詮釋學』使山海經中『夸父追日』的精神,不再那麼飄渺迷茫,也許訴說著人類問著『為什麼』的童年;那中國歷史上最早的一部天文曆算著作《周髀算經》則是『好奇後』的老成,似乎已經揭示了日月星辰的運行規律、四季更替、氣候變化甚至包涵南北有極晝夜相推的道理。可是誰又能一直持有年少時那顆天真好奇之心呢?
假使一個人果能站在前人學問的基石上,又天真好奇孜孜不倦,那就會如孔子在《論語‧子罕》:
後生可畏,焉知來者之不如今也。 四十、五十而無聞 ── ㄨㄣˊ陽關道 ──焉,斯亦不足畏也已。
,裡所說的一樣。甚至要能如下面所引的『一則故事』那樣
歐陽修,一向治學嚴謹,直至晚年,不減當初。他常將自己平生所寫的文章,清理出來進行修改,每字每句反覆推敲,甚是認真。為此,他整天辛苦勞累,有時直忙到深夜。夫人見他年歲已高,還如此盡心費神,恐其操勞過度,影響健康,十分擔心,目前制止。她關切地對丈夫說:『官人,何必如此用功,不惜貴體安康,為這些文字吃這樣多的苦頭,官人已年邁致仕(退休),難道還怕先生責難生氣嗎?』歐陽修回答說:『不怕先生生氣,只怕後生生譏』,『後生可畏耶!』
,活到老學到老。
── 英特乃者何耶?右尊讀之,是乃特英也,
乃特英者理念所鑄之網,祈立人願達人!! ──
─── 《後生可畏!?》
『概念』實難憑空而生!『創見』偶在夢中出現?
當人們在『夢中』喃喃自語,人們真的說了些什麼嗎?若是講有人『記下』了『夢中』所憶︰
pi@raspberrypi ~\displaystyle {\ce {{A}+ B <=> {A'}+ B'}}
\displaystyle {\text{affinity}}=\alpha [{{\ce {A}}}]^{a}[{{\ce {B}}}]^{b}
\displaystyle {\ce {{alcohol}+ acid <=> {ester}+ water}}
\displaystyle \alpha
\displaystyle \xi
\displaystyle \alpha (p-\xi )^{a}(q-\xi )^{b}=\alpha '(p'+\xi )^{a'}(q'+\xi )^{b'}
\displaystyle \xi
\displaystyle \left({\frac {dx}{dt}}\right)_{forward}=k(p-x)^{a}(q-x)^{b}
\displaystyle \left({\frac {dx}{dt}}\right)_{reverse}=k'(p'+x)^{a'}(q'+x)^{b'}
\displaystyle (p-x)^{a}(q-x)^{b}={\frac {k'}{k}}(p'+x)^{a'}(q'+x)^{b'}
\displaystyle {\mbox{affinity}}=\alpha [A][B]
\displaystyle k[A]_{\text{eq}}[B]_{\text{eq}}=k'[A']_{\text{eq}}[B']_{\text{eq}}
\displaystyle (p-\xi )(q-\xi )={\frac {k'}{k}}(p'+\xi )(q'+\xi )
\displaystyle v=\psi (k(p-x)(q-x)-k'(p'+x)(q'+x))
\displaystyle \psi
\displaystyle \xi
\displaystyle {\text{affinity}}=k[{{\ce {A}}}]^{\alpha }[{{\ce {B}}}]^{\beta }\dots
\displaystyle K={\frac {[S]^{\sigma }[T]^{\tau }\dots }{[A]^{\alpha }[B]^{\beta }\dots }}
\displaystyle r_{f}=k_{f}[A][B]
\displaystyle r_{f}
\displaystyle r_{f}
\displaystyle \mathrm {{C}_{12}{H}_{22}{O}_{11}(s)} \rightleftharpoons \mathrm {{C}_{12}{H}_{22}{O}_{11}(aq)}
\displaystyle K={\frac {\left\{\mathrm {{C}_{12}{H}_{22}{O}_{11}} (aq)\right\}}{\left\{\mathrm {{C}_{12}{H}_{22}{O}_{11}} (s)\right\}}}
\displaystyle K_{s}=\left[\mathrm {{C}_{12}{H}_{22}{O}_{11}} (aq)\right]
\displaystyle \mathrm {CaSO} _{4}(s)\rightleftharpoons {\mbox{Ca}}^{2+}(aq)+{\mbox{SO}}_{4}^{2-}(aq)
\displaystyle K={\frac {\left\{{\mbox{Ca}}^{2+}(aq)\right\}\left\{{\mbox{SO}}_{4}^{2-}(aq)\right\}}{\left\{{\mbox{CaSO}}_{4}(s)\right\}}}
\displaystyle K_{\mathrm {sp} }=\left[{\mbox{Ca}}^{2+}(aq)\right]\left[{\mbox{SO}}_{4}^{2-}(aq)\right].
\displaystyle {\sqrt {K_{\mathrm {sp} }}}={\sqrt {4.93\times 10^{-5}}}=7.02\times 10^{-3}=\left[{\mbox{Ca}}^{2+}\right]=\left[{\mbox{SO}}_{4}^{2-}\right].
\displaystyle \mathrm {Ca(OH)_{2}} (s)\rightleftharpoons {\mbox{Ca}}^{2+}(aq)+{\mbox{2OH}}^{-}(aq)
\displaystyle \mathrm {A} (s)\rightleftharpoons {\mbox{xB}}^{p+}(aq)+{\mbox{yC}}^{q-}(aq)
\displaystyle {\sqrt[{n}]{K_{\mathrm {sp} } \over {x^{x}\cdot y^{y}}}}={C \over M_{M}}$
其中:
- n是電離方程右邊的總計量數(對上例,x+y),無量綱;
- x是所有陽離子的總計量數,無量綱;
- y是所有陰離子的總計量數,無量綱;
- Ksp是溶度積,(mol/kg)n;
- C是化合物 A的溶解度(A的質量比溶液的質量),無量綱;
- MM是化合物 A的摩爾質量,kg/mol。
上述方程假設電離過程發生在純溶劑(無同離子效應發生),亦不存在絡合和水解(即溶液中只存在Bp+和Cq-),且濃度小到離子活度可被認為等於1。
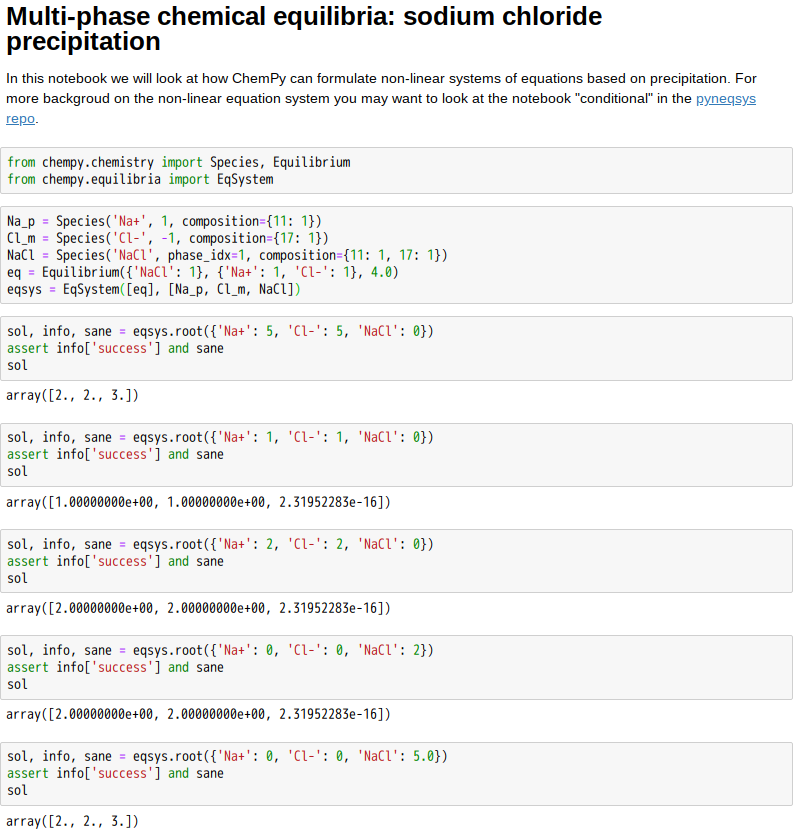
焉不能互動求『解』呦◎