阿難。如汝所言四大和合。發明世間種種變化。阿難。若彼大性體非和合。則不能與諸大雜和。猶如虛空。不和諸色。若和合者。同於變化。始終相成。生滅相續。生死死生。生生死死。如旋火輪。未有休息。阿難。如水成冰。冰還成水。汝觀地性。麤為大地。細為微塵。至鄰虛塵。析彼極微色邊際相。七分所成。更析鄰虛。即實空性。阿難。若此鄰虛。析成虛空。當知虛空。出生色相。汝今問言。由和合故。出生世間諸變化相 。汝且觀此一鄰虛塵。用幾虛空和合而有。不應鄰虛合成鄰虛。又鄰虛塵。析入空者。用幾色相 。合成虛空。若色合時。合色非空。若空合時。合空非色。色猶可析。空云何合。汝元不知。如來藏中。性色真空。性空真色。清淨本然。周遍法界。隨眾生心。應所知量。循業發現。世間無知。惑為因緣及自然性。皆是識心分別計度。但有言說。都無實義。
若問光子有大小嗎?以波動性論耶?用粒子性說乎?既是時空中之存在,如何沒有大小!既然沒有大小,又怎麼講可以量子生滅呢!解析大地粗細,以至於鄰虛塵,可知性色真空、性空真色 的乎!!卡西米爾議論真空漲落,加總無量光子能量 ,竟得有限量的耶??假使真知光子本來面目,得見如來的吧??!!
─── 《光的世界︰【□○閱讀】顯微鏡《上》》
雙手推開窗前月!一石驚破井中天?
莫非『日光白』所說事?
Presence of Light
Light provides necessary activation energy to the starting materials, therefore, most of the reactions becomes faster in the presence of light
因此娑婆世界『變化多』!
一時巽入『鄰虛塵』︰
Dynamic equilibrium
In chemistry, a dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state. In thermodynamics, a closed system is in thermodynamic equilibrium when reactions occur at such rates that the composition of the mixture does not change with time. Reactions do in fact occur, sometimes vigorously, but to such an extent that changes in composition cannot be observed. Equilibrium constants can be expressed in terms of the rate constants for elementary reactions.
Examples
In a new bottle of soda the concentration of carbon dioxide in the liquid phase has a particular value. If half of the liquid is poured out and the bottle is sealed, carbon dioxide will leave the liquid phase at an ever-decreasing rate and the partial pressure of carbon dioxide in the gas phase will increase until equilibrium is reached. At that point, due to thermal motion, a molecule of CO2 may leave the liquid phase, but within a very short time another molecule of CO2 will pass from the gas to the liquid, and vice versa. At equilibrium the rate of transfer of CO2 from the gas to the liquid phase is equal to the rate from liquid to gas. In this case, the equilibrium concentration of CO2 in the liquid is given by Henry’s law, which states that the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid.[1] This relationship is written as
where k is a temperature-dependent constant, p is the partial pressure and c is the concentration of the dissolved gas in the liquid Thus the partial pressure of CO2 in the gas has increased until Henry’s law is obeyed. The concentration of carbon dioxide in the liquid has decreased and the drink has lost some of its fizz.
Henry’s law may be derived by setting the chemical potentials of carbon dioxide in the two phases to be equal to each other. Equality of chemical potential defines chemical equilibrium. Other constants for dynamic equilibrium involving phase changes, include partition coefficient and solubility product. Raoult’s law defines the equilibrium vapor pressure of an ideal solution
Dynamic equilibrium can also exist in a single-phase system. A simple example occurs with acid-base equilibrium such as the dissociation of acetic acid, in aqueous solution.
- CH3CO2H ⇌
CH3CO2− + H+
At equilibrium the concentration quotient, K, the acid dissociation constant, is constant (subject to some conditions)
In this case, the forward reaction involves the liberation of some protons from acetic acid molecules and the backward reaction involves the formation of acetic acid molecules when an acetate ion accepts a proton. Equilibrium is attained when the sum of chemical potentials of the species on the left-hand side of the equilibrium expression is equal to the sum of chemical potentials of the species on the right-hand side. At the same time the rates of forward and backward reactions are equal to each other. Equilibria involving the formation of chemical complexes are also dynamic equilibria and concentrations are governed by the stability constants of complexes.
Dynamic equilibria can also occur in the gas phase as, for example, when nitrogen dioxide dimerizes.
In the gas phase, square brackets indicate partial pressure. Alternatively, the partial pressure of a substance may be written as P(substance).[2]
Relationship between equilibrium and rate constants
In a simple reaction such as the isomerization:
there are two reactions to consider, the forward reaction in which the species A is converted into B and the backward reaction in which B is converted into A. If both reactions are elementary reactions, then the rate of reaction is given by[3]
where kf is the rate constant for the forward reaction and kb is the rate constant for the backward reaction and the square brackets, [..] denote concentration . If only A is present at the beginning, time t=0, with a concentration [A]0, the sum of the two concentrations, [A]t and [B]t, at time t, will be equal to [A]0.
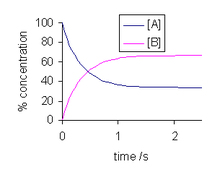
% concentrations of species in isomerization reaction. kf = 2 s−1, kr = 1 s−1
The solution to this differential equation is
and is illustrated at the right. As time tends towards infinity, the concentrations [A]t and [B]t tend towards constant values. Let t approach infinity, that is, t→∞, in the expression above:
In practice, concentration changes will not be measurable after ![]() . Since the concentrations do not change thereafter, they are, by definition, equilibrium concentrations. Now, the equilibrium constant for the reaction is defined as
. Since the concentrations do not change thereafter, they are, by definition, equilibrium concentrations. Now, the equilibrium constant for the reaction is defined as
It follows that the equilibrium constant is numerically equal to the quotient of the rate constants.
In general they may be more than one forward reaction and more than one backward reaction. Atkins states[4] that, for a general reaction, the overall equilibrium constant is related to the rate constants of the elementary reactions by
遊戲範例『文字』間!!
Copper(II)amine complexes (speciation)

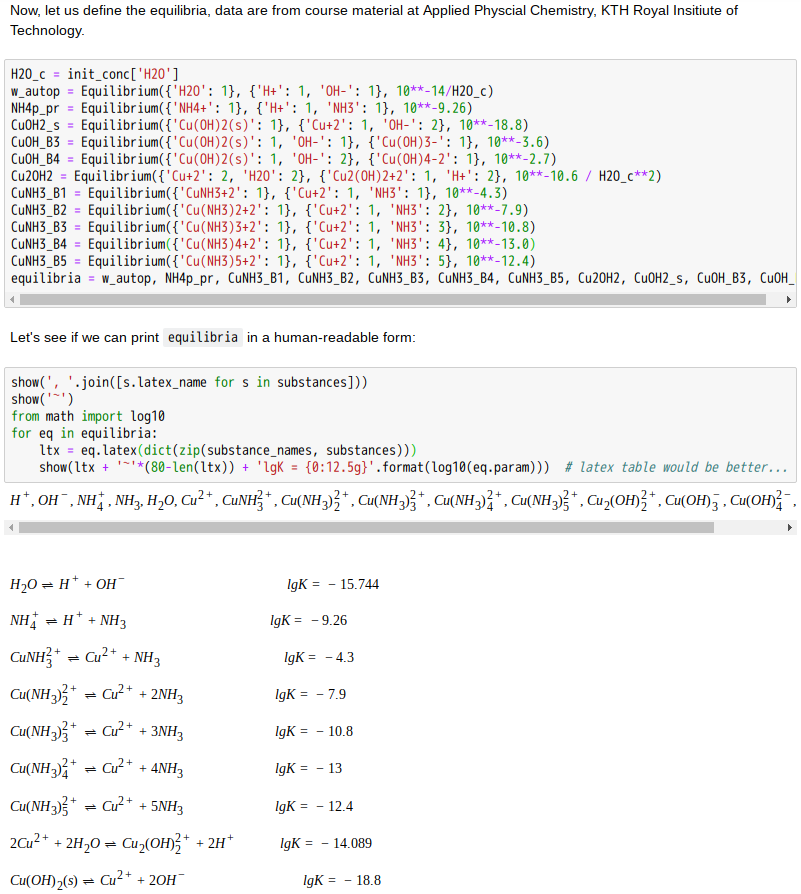
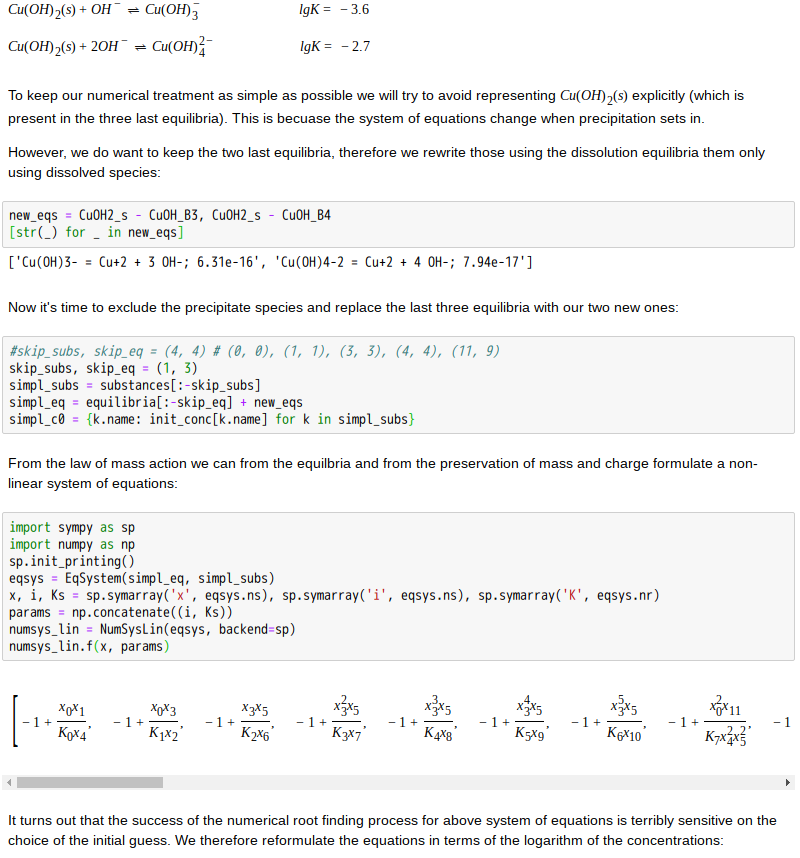
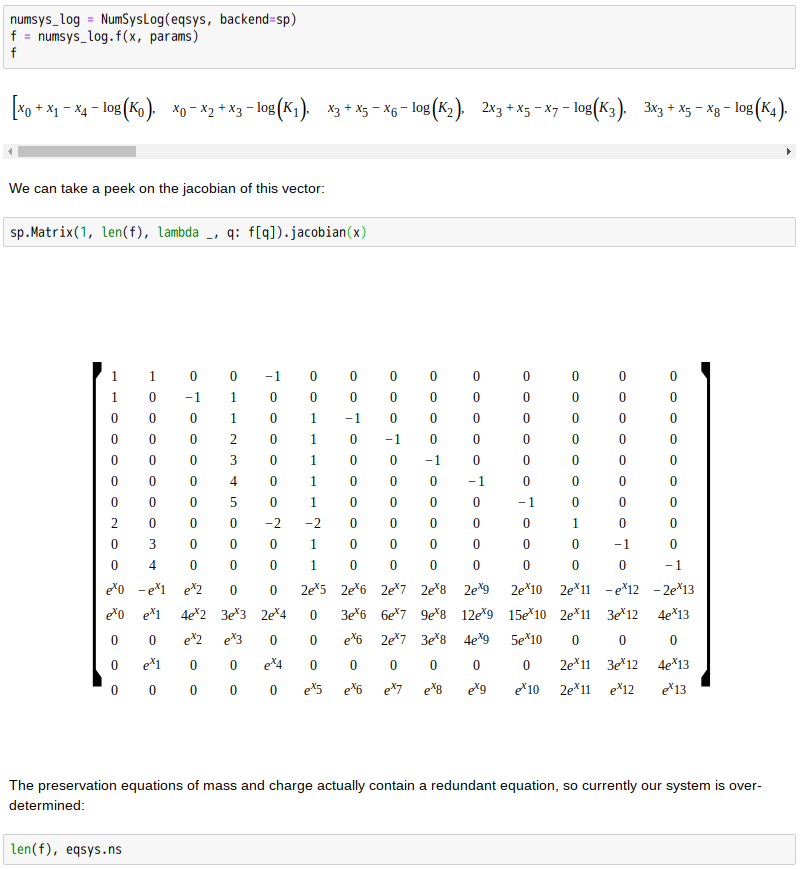
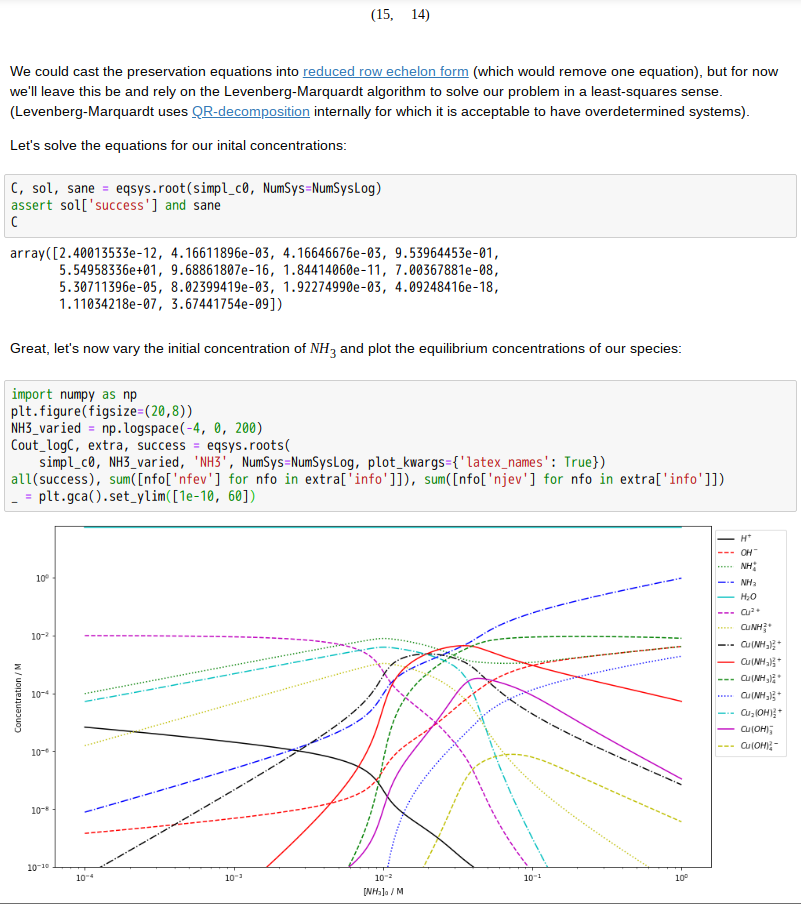
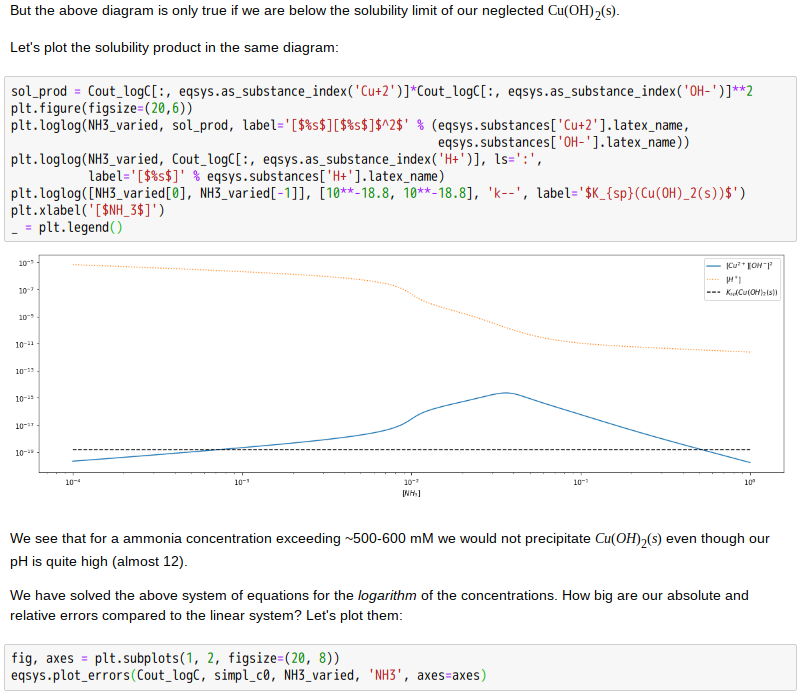
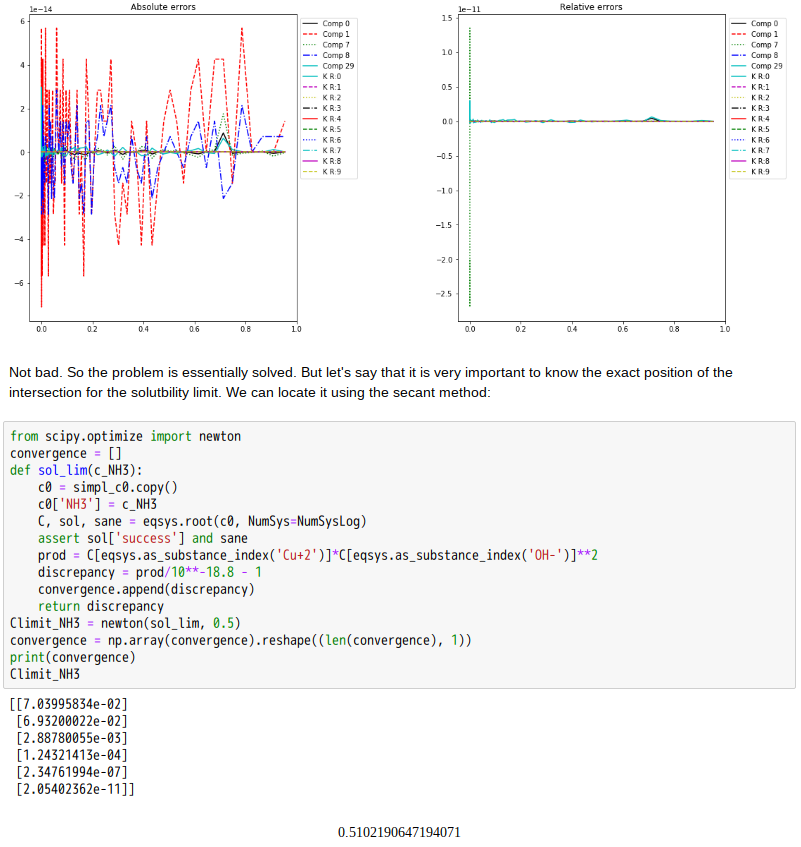
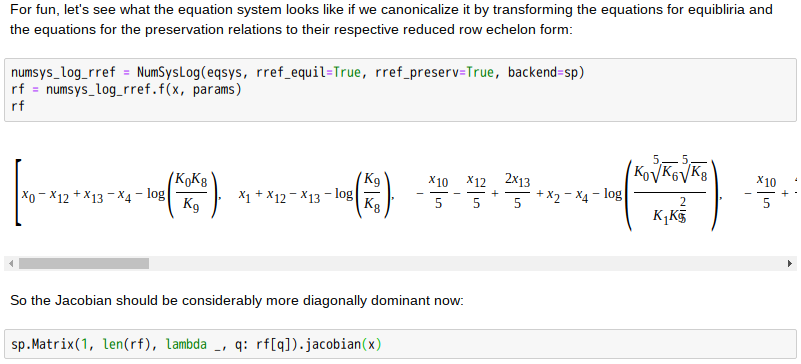
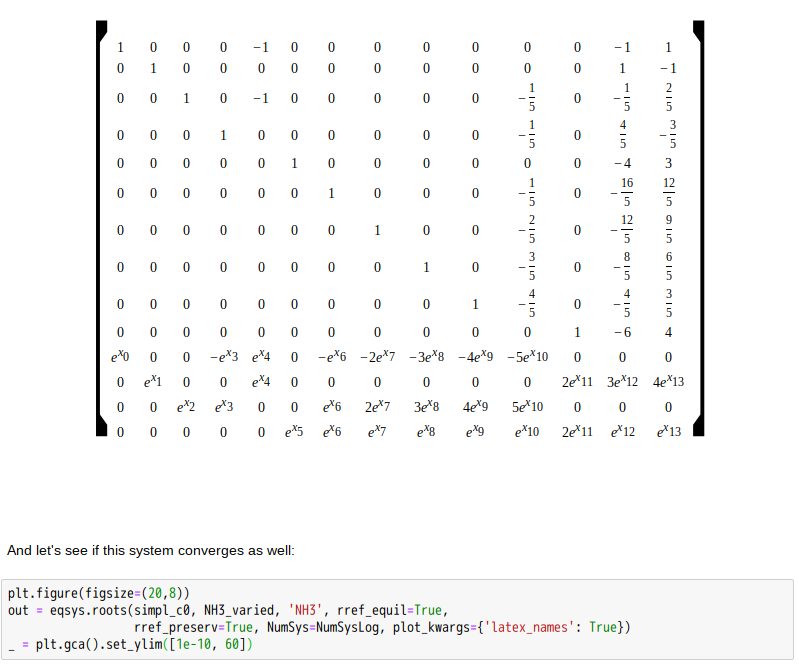
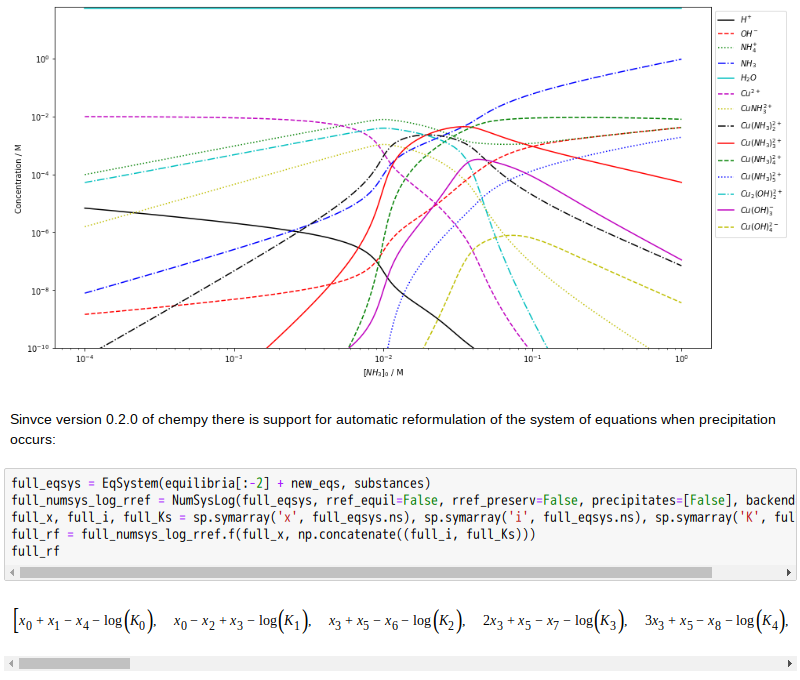
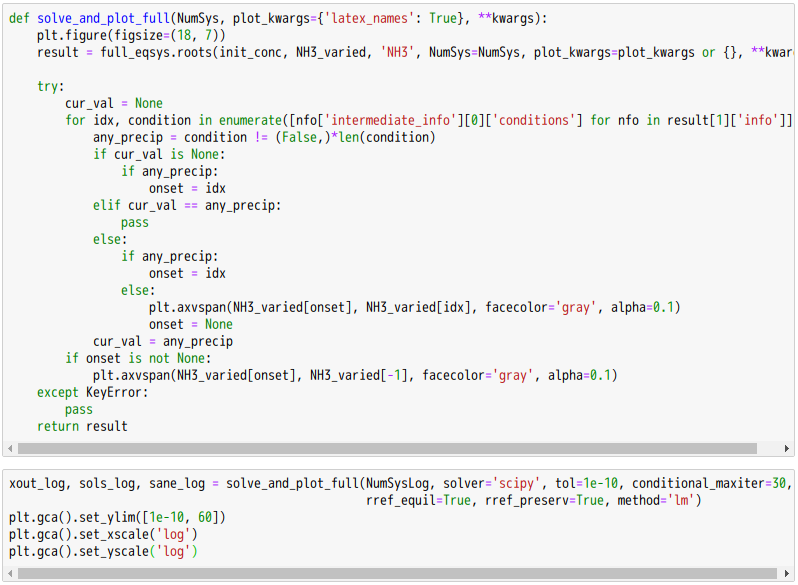
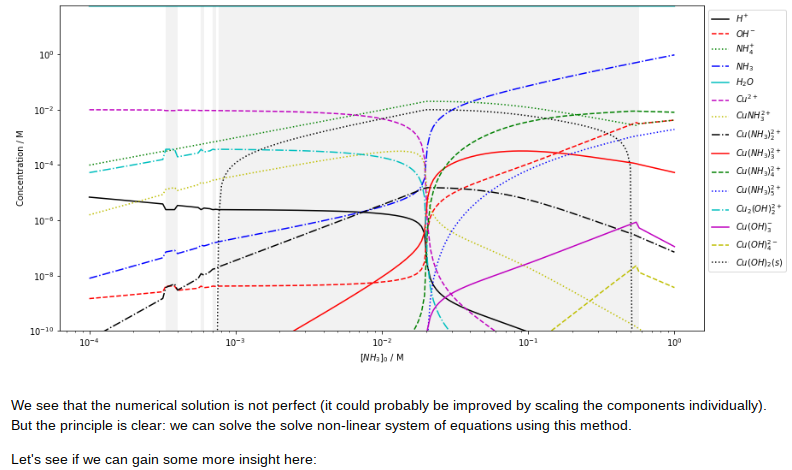
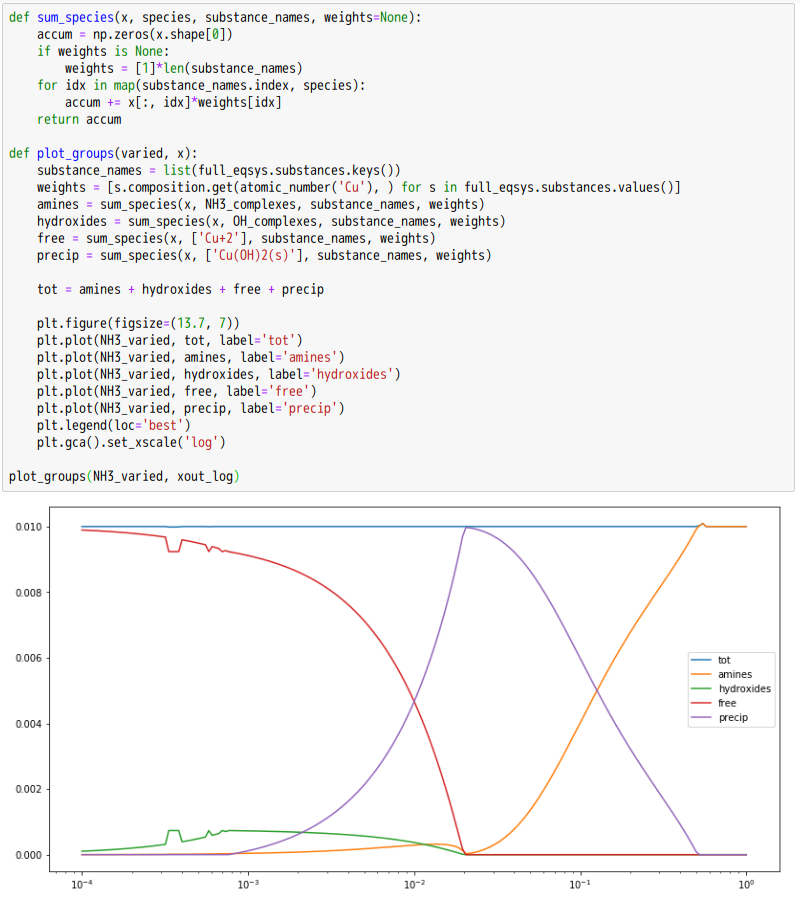
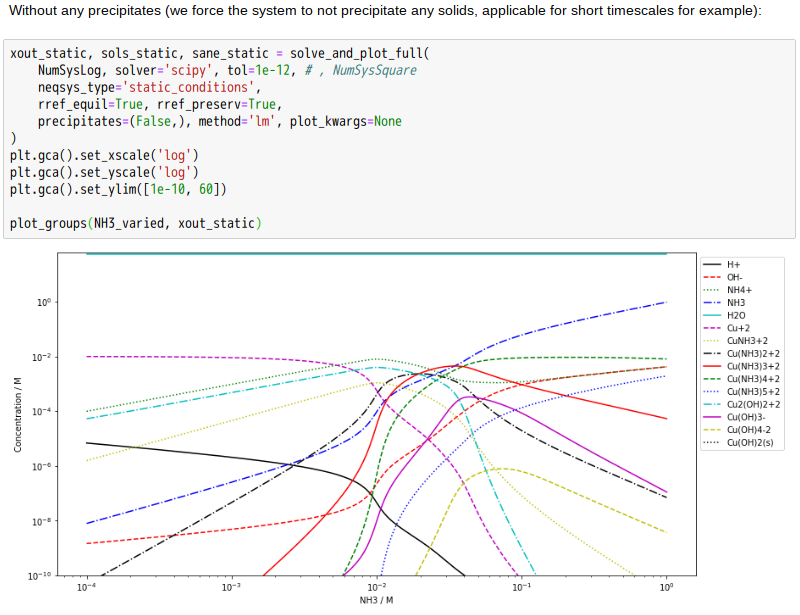
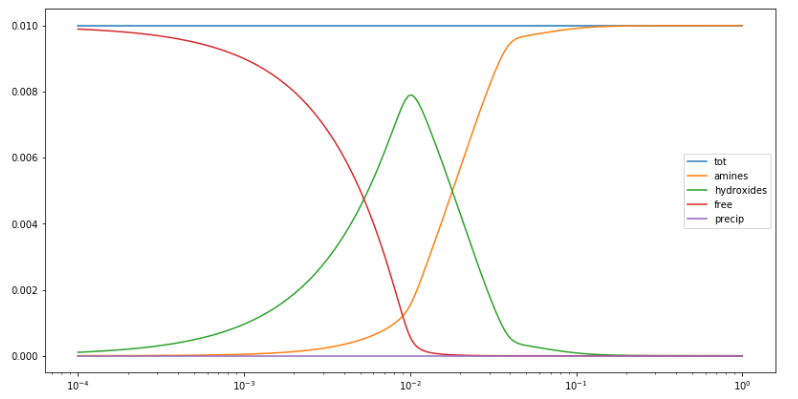
忽憶『指令』無從來?★
只得先啃『原始』碼
Source code for chempy.chemistry
# -*- coding: utf-8 -*-
from __future__ import (absolute_import, division, print_function)
from collections import OrderedDict, defaultdict
from functools import reduce
from itertools import chain
from operator import mul, add
import math
import warnings
from .util.arithmeticdict import ArithmeticDict
from .util._expr import Expr
from .util.periodic import mass_from_composition
from .util.parsing import (
formula_to_composition, to_reaction,
formula_to_latex, formula_to_unicode, formula_to_html
)
from .units import default_units, is_quantity, unit_of, to_unitless
from ._util import intdiv
from .util.pyutil import deprecated, DeferredImport, ChemPyDeprecationWarning
ReactionSystem = DeferredImport('chempy.reactionsystem', 'ReactionSystem',
[deprecated(use_instead='chempy.ReactionSystem')])
[docs]class Substance(object):
""" Class representing a chemical substance
Parameters
----------
name : str
charge : int (optional, default: None)
Will be stored in composition[0], prefer composition when possible.
latex_name : str
unicode_name : str
html_name : str
composition : dict or None (default)
Dictionary (int -> number) e.g. {atomic number: count}, zero has special
meaning (net charge). Avoid using the key 0 unless you specifically mean
net charge. The motivation behind this is that it is easier to track a
net-charge of e.g. 6 for U(VI) than it is to remember that uranium has 92
electrons and use 86 as the value).
data : dict
Free form dictionary. Could be simple such as ``{'mp': 0, 'bp': 100}``
or considerably more involved, e.g.: ``{'diffusion_coefficient': {\
'water': lambda T: 2.1*m**2/s/K*(T - 273.15*K)}}``.
Attributes
----------
mass
Maps to data['mass'], and when unavailable looks for ``formula.mass``.
attrs
A tuple of attribute names for serialization.
composition : dict or None
Dictionary mapping fragment key (str) to amount (int).
data
Free form dictionary.
Examples
--------
>>> ammonium = Substance('NH4+', 1, 'NH_4^+', composition={7: 1, 1: 4},
... data={'mass': 18.0385, 'pKa': 9.24})
>>> ammonium.name
'NH4+'
>>> ammonium.composition == {0: 1, 1: 4, 7: 1} # charge represented by key '0'
True
>>> ammonium.data['mass']
18.0385
>>> ammonium.data['pKa']
9.24
>>> ammonium.mass # mass is a special case (also attribute)
18.0385
>>> ammonium.pKa
Traceback (most recent call last):
...
AttributeError: 'Substance' object has no attribute 'pKa'
>>> nh4p = Substance.from_formula('NH4+') # simpler
>>> nh4p.composition == {7: 1, 1: 4, 0: 1}
True
>>> nh4p.latex_name
'NH_{4}^{+}'
"""
attrs = (
'name', 'latex_name', 'unicode_name', 'html_name',
'composition', 'data'
)
...
再論其餘說
沉澱
沉澱在化學上指從溶液中析出固體物質的過程;也指在沉澱過程中析出的固體物質。事實上沉澱多為難溶物(20°C時溶解度<0.01g) 。在化學實驗和生產中廣泛應用沉澱方法進行物質的分離。
在水處理中指懸浮物在水中下沉,是懸浮物和水在密度上的差異形成的。
原因
化學中沉澱的產生是由於化學反應而生成溶解度較小的物質,或者由於溶液的濃度大於該溶質的溶解度所引起的;
標識
通常在化學反應方程式中沉澱會被標上「↓」,如:
從液相中產生可分離固相物的過程
!。
![Rendered by QuickLaTeX.com \displaystyle [A]_{t}={\frac {k_{b}+k_{f}e^{-\left(k_{f}+k_{b}\right)t}}{k_{f}+k_{b}}}[A]_{0}](http://www.freesandal.org/wp-content/ql-cache/quicklatex.com-cac68f84b28d696c739868305f5a25b7_l3.png)
![Rendered by QuickLaTeX.com \displaystyle K={\frac {{\frac {k_{f}}{k_{f}+k_{b}}}[A]_{0}}{{\frac {k_{b}}{k_{f}+k_{b}}}[A]_{0}}}={\frac {k_{f}}{k_{b}}}](http://www.freesandal.org/wp-content/ql-cache/quicklatex.com-9204d6f5f08ad6a099e7a0d0284744df_l3.png)